molecular shape of ethanol|C2H5OH Lewis Structure, Molecular Geometry : Tagatay Molecular weight: 46.0684. IUPAC Standard InChI: InChI=1S/C2H6O/c1-2-3/h3H,2H2,1H3. IUPAC Standard InChIKey: LFQSCWFLJHTTHZ-UHFFFAOYSA-N. CAS Registry .
Rep. LRay Villafuerte and three colleagues call on President Marcos Jr. to revoke Evenchance Gaming Corp.’s STL franchise, citing consistent violations of STL rules and national laws. The lawmakers express full support for the pleas of CamSur residents, presenting evidence of Evenchance’s alleged use of STL as a front for illegal .
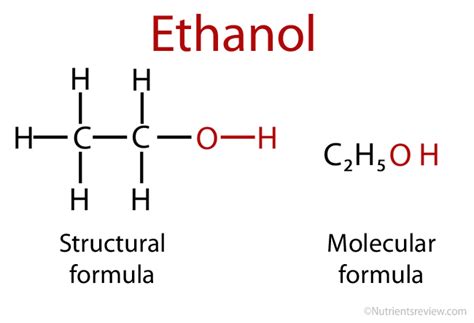
molecular shape of ethanol,An explanation of the molecular geometry for the C2H5OH (Ethanol) including a description of the C2H5OH bond angles.
Ethanol is a 2-carbon alcohol. Its molecular formula is CH3CH2OH. The structure of the molecule of ethanol is CH3−CH2−OH (an ethyl group linked to a hydroxyl group), which indicates that the carbon of a methyl group (CH3−) is attached to the carbon of a methylene group (−CH2–), which is attached to the oxygen of a hydroxyl group (−OH). It is a constitutional isomer of dimethyl ether. Ethanol is .

ethanol, a member of a class of organic compounds that are given the general name alcohol s; its molecular formula is C 2 H 5 OH. Ethanol is an important industrial chemical; it is .Ethanol | CH3CH2OH or C2H6O | CID 702 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, . Ethanol (C2H5OH) Lewis structure, molecular geometry or shape, electron geometry, bond angle, hybridization, formal charges, isomers, polar or nonpolar. C 2 H 5 OH is the chemical formula for .Molecular weight: 46.0684. IUPAC Standard InChI: InChI=1S/C2H6O/c1-2-3/h3H,2H2,1H3. IUPAC Standard InChIKey: LFQSCWFLJHTTHZ-UHFFFAOYSA-N. CAS Registry . Let us look at how we can find the molecular geometry of ethanol and how it appears to be in plane space. The best way to find the molecular geometry of any compound is by using the VSEPR or the .
When a molecule or polyatomic ion has only one central atom, the molecular structure completely describes the shape of the molecule. Larger molecules do not have a single central atom, but are connected by a . Ethyl Alcohol Formula & Structure. Structural Formula. C 2 H 6 O. ethyl alcohol. ethanol. Molecular Model. Jmol._Canvas2D (JSmol) "jmolApplet0" [x] .However, when the molecules are mixed, new hydrogen bonds are formed between water molecules and ethanol molecules. The energy released when these new hydrogen bonds form approximately compensates for the energy needed to break the original interactions. In addition, there is an increase in the disorder of the system, an increase in entropy.
The strengths of London dispersion forces also depend significantly on molecular shape because shape determines how much of one molecule can interact with its neighboring molecules at any given time. For example, part (b) in Figure \(\PageIndex{4}\) . Hydrogen bonding can occur between ethanol molecules, although .Total valence electrons pairs. Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells. Total electron pairs are determined by dividing the number total valence electrons by two.For, ethanol, total pairs of electrons are ten in their valence shells. Center atom of ethanol. There are several guidelines to identify the center atom of a .
For example, the molecular formula of butane is \(C_4H_{10}\), and the molecular formula of ethanol is \(C_2H_6O\). Molecular formulae are very rarely used in organic chemistry, because they do not give useful information about the bonding in the molecule. . Notice that the way the methane is drawn bears no resemblance to the actual shape of .Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula CH 3 CH 2 OH.It is an alcohol, with its formula also written as C 2 H 5 OH, C 2 H 6 O or EtOH, where Et stands for ethyl.Ethanol is a volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent .
IUPAC Standard InChIKey: LFQSCWFLJHTTHZ-UHFFFAOYSA-N Copy CAS Registry Number: 64-17-5 Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using . The molecular shape of a molecule is not the same as its molecular geometry if the difference between the no. of bond pairs electrons and the steric number is not zero, i.e the molecule contains non-zero lone pairs in it. . and lewis structure of ethanol. CH3OH Polarity. If the charge distribution between two atoms is unequal or .
The H-bonding of ethanol results in a liquid for cocktails at room temperature, while the weaker dipole-dipole of the dimethylether results in a gas a room temperature. In the last example, we see the three IMFs compared directly to illustrate the relative strength IMFs to boiling points. . Molecular shape, and the ability of a molecule to . The oxygen atom, with two bonds and two lone pairs, has a bent molecular shape and a tetrahedral electron geometry. Polarity of Ethanol. Ethanol is a polar molecule, primarily due to the presence of the hydroxyl group (-OH). The difference in electronegativity between oxygen and hydrogen in this group creates a polar bond, .
Ethanol is a chemical compound that is commonly used as a solvent, fuel, and in the production of alcoholic beverages. Its molecular formula is C2H5OH, and it consists of two carbon atoms, six hydrogen atoms, and one oxygen atom. The Lewis dot structure of ethanol shows the arrangement of these atoms and their valence .Why Do Molecular Compounds Differ in Shape? VESPR (valence shell electron pair repulsion) theory states that negatively charged electrons repel one another. The forces that make them repel one another distorts the bond angles, thus affecting the shape of molecules. The shapes of molecules in a compound are dictated by the VESPR theory.C2H5OH Lewis Structure, Molecular Geometry As a result, the molecule of ethanol gives non zero dipole moment and becomes a polar molecule. Ethanol is a well-known substance that is majorly consumed by humans all over the world in the .molecular shape of ethanolethanol, a member of a class of organic compounds that are given the general name alcohols; its molecular formula is C 2 H 5 OH. Ethanol is an important industrial chemical; it is used as a solvent , in the synthesis of other organic chemicals, and as an additive to automotive gasoline (forming a mixture known as a gasohol ).
{ "name":"jmolApplet0_object","applet":true,"documentBase":"https://www.chem.purdue.edu/jmol/molecules/ch3ch2oh.html","platform":"J.awtjs2d.Platform","fullName . Ideally, a model should reflect not only the size and shape of the molecule it represents but also the flexibility of the molecule. By this we mean that. Figure 2-3: CPK space-filling models of organic compounds. it should simulate the type of motions available to the molecule, particularly bond rotation.

All mass spectra in this site (plus many more) are available from the NIST/EPA/NIH Mass Spectral Library. Please see the following for information about the library and its accompanying search program.A computational study of (ethanol) n-water, n = 1 to 5 heteroclusters was carried out employing the B3LYP/6-31+G(d) approach. The molecular (MO) and atomic (AO) orbital analysis and the .molecular shape of ethanol C2H5OH Lewis Structure, Molecular GeometryThe Ethanol Molecule -- Chemical and Physical Properties . The chemical compound ethanol, also known as ethyl alcohol or grain alcohol, is the bio-alcohol found in alcoholic beverages.When non-chemists refer to "alcohol", they almost always mean ethanol.It is also increasingly being used as a fuel (usually replacing or complementing gasoline).
molecular shape of ethanol|C2H5OH Lewis Structure, Molecular Geometry
PH0 · Ethyl Alcohol Formula & Structure
PH1 · Ethanol (C2H5OH) Lewis structure, molecular
PH2 · Ethanol
PH3 · C2H5OH Lewis Structure, Molecular Geometry
PH4 · C2H5OH Lewis Structure, Molecular Geometry
PH5 · C2H5OH (Ethanol): Molecular Geometry and Bond Angles
PH6 · 5.2: Molecular Shape